 In a previous article, I explained how epigenetic mechanisms regulate the structure and function of DNA.1 Specifically, I showed how small molecules can be attached to the DNA itself or added to the histone proteins that the DNA is packaged with. These are known as DNA methylation or histone modifications, respectively. The modifications are monitored and performed dynamically according to the needs of the cell and adaptively in response to environmental changes. Furthermore, these epigenetic modifications can be heritable to give the organism’s offspring an adaptive advantage.
In a previous article, I explained how epigenetic mechanisms regulate the structure and function of DNA.1 Specifically, I showed how small molecules can be attached to the DNA itself or added to the histone proteins that the DNA is packaged with. These are known as DNA methylation or histone modifications, respectively. The modifications are monitored and performed dynamically according to the needs of the cell and adaptively in response to environmental changes. Furthermore, these epigenetic modifications can be heritable to give the organism’s offspring an adaptive advantage.
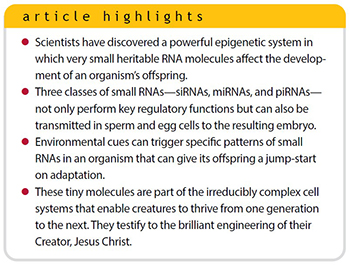
In addition to DNA methylation and histone modifications, scientists have discovered a powerful epigenetic system that operates on top of the genetic code in a very different way from being chemically attached to the chromatin itself. (DNA complexed with histones is called chromatin.) This amazing epigenetic paradigm is associated with very small RNA molecules that are heritable and that profoundly and adaptively affect the development of an organism’s offspring.
The Search Engine of the Genome
One of the best ways to describe how small RNAs work in the cell is to use the analogy of an internet search engine. When a search engine is pulled up in your web browser, you can type in a small bit of text, hit enter, and then get a hit wherever the text matches up. For example, you could enter “for God so loved the world,” and it would immediately pull up the Bible verse John 3:16.
Cells also have powerful search engines that are specialized protein machines. These protein machines take a small 22- to 30-letter piece of RNA as their search engine input, which is a string of nucleotide text associated with part of a specific gene in the genome. The small piece of RNA binds to a specialized protein, and then off it goes to find a match. When a match is made to a corresponding piece of code, the gene itself or even the RNA product of the gene is bound by the RNA-protein complex, silenced, and turned off.
What is even more amazing is that these little RNAs can also be transmitted in the sperm and egg and affect the development of the resulting embryo.2,3 The effects of these small, heritable RNAs can persist in an organism’s offspring for up to four generations. Furthermore, environmental cues detected by an organism can trigger specific patterns of small RNAs that will heritably prime the future offspring for a jump-start on adaptation.
While their function is similar, the search engine capacities in the cell are considerably more advanced and variable than internet search engines. The specific type of the small RNA-based search engine scheme has three main systems: RNA interference (RNAi, also called small interfering RNA or siRNA), microRNA (miRNA), and piwi-interacting RNA (piRNA). I will discuss each system briefly and then review their importance as cleverly engineered adaptive control systems.
The RNA Interference System
Scientists have known since the 1980s that the injection of RNA molecules into cells corresponding to certain genes of interest can affect their expression levels, even silencing the genes. Surprisingly, this can be achieved using either a sense or anti-sense form of the RNA molecule.3 In the late 1990s and early 2000s, it was shown that the injection of double-stranded RNA molecules corresponding to specific genes into the cells of trypanosomes (single-cell eukaryotic parasites), plants, fruit flies, and mammals could cause the specified genes to be silenced.3
Researchers then began to genetically dissect and examine the proteins involved in this mysterious cellular process. In this pathway, known as RNA interference (RNAi), one of the key players is a piece of protein machinery called an RNA-dependent RNA polymerase that makes RNA copies based on an RNA template.3 Additionally, many other components of the “RNAi machinery” were identified and documented in plants, invertebrates, and vertebrates.3
The RNAi pathway as we currently understand it can be described in the following simplified model. The double-stranded RNAs in the cell are recognized and bound by a group of proteins called double-stranded RNA binding proteins. These then interact with an enzyme called dicer that chops the double-stranded RNAs into small pieces that are about 20 to 23 nucleotides long. These small RNAs are copied and amplified by the RNA polymerase mentioned above and can then act as small interfering RNAs or siRNAs.
These newly copied RNAs are unwound, and one of the two strands acts as the guide RNA or search engine input, as mentioned earlier. This RNA is loaded into the search engine mechanism, which is a protein machine called the RNA-induced silencing complex, or RISC. The RISC complex finds a complementary RNA that matches the guide RNA and cleaves it with the assistance of an Argonaute protein, which is the catalytic component of the RISC complex. The end result is that messenger RNAs are selectively targeted, degraded, and removed—effectively silencing the products of specified genes.
Since there are a range of RNAi pathways and many variants in the RISC complex and Argonaute proteins, these systems in the cell operate at several different levels. In plants, insects, and vertebrates, RNAi mechanisms are known to directly influence the methylation of DNA in the genome. In a previous article, I explained how the addition of methyl tags on the cytosine molecules in DNA can either shut down or activate genetic activity.1 At present, it’s believed that the RISC complex can recruit the methylation machinery (methyltransferase enzyme) and that the guide RNA provides the targeting specificity.
Both small RNAs and epigenetic states in the DNA are dynamically produced based on environmental cues detected by the organisms. Furthermore, these factors can be inherited over several generations. Thus, the potential effect of priming future generations of creatures to have an advantage in adaptation is profound. This adaptive effect begins during the development of the embryo and is regulated by both small RNAs and epigenetic states. In this respect, siRNAs play important roles in not only organism development but also stress responses, pathogen interactions, DNA damage repair systems, and 3-D chromosome structure in the nucleus.
In another article, I showed how transposable elements (TEs) are key, designed features of the genome that provide a host of functions in adaptation.4 Not surprisingly, there is a strong connection between TEs and siRNAs. As it turns out, many siRNAs are encoded by sequences within transposable elements. Conversely, many siRNAs also regulate the activity and expression of sequences within TEs. In addition, siRNAs have been found to regulate the overall activity of TEs, including their transposition (movement in the genome).
MicroRNAs
MicroRNAs (miRNAs) are another class of small, non-coding RNAs that play a large and important role in regulating gene expression in the cell.5 The majority of miRNAs are copied (transcribed) from DNA sequences into long primary miRNAs, processed into precursor miRNAs, and then finally processed into mature miRNAs that are about 19 to 25 nucleotides long. In most cases, miRNAs interact with the tail end of a targeted, long protein-coding RNA in a region called the 3´ untranslated region (3´ UTR). This miRNA targeting often results in the target mRNA’s degradation or its repression from being used to create a protein. However, the interaction of miRNAs with other regions of an RNA transcript, including its leader sequence (called a 5´ untranslated region), is also being documented.
While most miRNA action occurs outside the nucleus of the cell by regulating the translation of RNAs in the cytoplasm, some miRNAs can also regulate transcription of genes in the nucleus.5 This interaction of miRNAs with their target genes on chromosomes is a dynamic process that depends on many factors, including the quantities of both the miRNAs and the target mRNAs being transcribed. And since the targeting of miRNAs to an mRNA contains a measure of imperfect binding, the affinity of the miRNA-mRNA interaction also plays an important role.
Not only do miRNAs function in the cell, but they can also be secreted outside the cell in specialized lipid capsules called vesicles.5 These are suspended in extracellular fluids and transported to target cells in other parts of the organ or body. In this role, extracellular miRNAs function as specialized chemical messengers that mediate cell-to-cell communication systems.
While miRNAs play a huge role in the biology of individual organisms, they are also heritable in both egg and sperm and thus play a major role in embryo development and the expression of adaptive traits in the next generation. In this respect, a 2021 study revealed new insights into the role of mRNAs and miRNAs in host gut tissue functions in Japanese quail.6 The study found that miRNAs played a major role in gut phosphorus utilization and other related traits in terms of the genetic regulation and inheritance of gut-related gene expression in a complex association with gut microbiome components. In other words, the inheritance of specific miRNAs gave offspring a jump-start on exploiting nutritional components of the bird’s environmental food supply.
Another study showed that sperm miRNAs in mice restructured early embryonic gene expression profiles during the development of core neuronal circuits, which affected mental health to create depression-like behaviors.7 Overall, the findings revealed that sperm miRNAs play a causal role in the inheritance of depression. The study results not only provide insight into a possible mechanism underlying susceptibility to depression but also into the important role small RNAs play in epigenetic heritability, a factor that significantly impacts the course of embryonic brain development.
Piwi-Interacting RNAs
Piwi-interacting RNAs (piRNAs) are a fairly recently discovered group of small RNAs that are 26 to 30 nucleotides in length and bind to a class of biomolecules called Piwi proteins.8 These short RNAs were originally discovered in germ-line cells (leading to sperm and egg cells) but are now also being found to be important genome regulators in many other bodily cells (somatic cells). These small RNAs are biologically significant because they regulate gene expression, transposable element (TE) activity, epigenetic programming, DNA rearrangements, gene transcript production, and translational control (protein production) of gene transcripts in the cytoplasm.8 A large body of research is revealing that the dysregulation of piRNAs can cause epigenetic changes that contribute to diverse health issues such as cancer, heart disease, nervous system disorders, retinal disease, and reproductive system problems.9
A large proportion of piRNAs are produced from specialized genetic regions on chromosomes called piRNA clusters.8 While these regions provide the genetic information to code for multiple piRNAs, the entire cluster is controlled as if it were a single gene. A large mRNA is copied from the cluster, and the resulting transcript is processed into individual piRNAs that are then bound to Piwi proteins. Some of these piRNAs work in the cell’s nucleus to recruit and direct DNA methyltransferases to methylate cytosines1—a form of epigenetic modification of DNA that downregulates genes or silences the activity of TEs. Other piRNAs do their work in the cell’s cytoplasm to regulate the translation (making of a protein from messenger RNA) of specific genes.
Clearly, these piRNAs are yet one more regulatory feature of an organism’s response to dynamically adapt to its environment and regulate the activity of the genome. Furthermore, these piRNAs are intimately connected to the embryonic development of an organism, where they regulate the activity of TEs. In fruit flies, piRNAs have been shown to be intergenerationally inherited through the maternal lineage.9 In this respect, the piRNAs specify TE locations on chromosomes in the developing embryo to be silenced. These maternally inherited piRNAs enter somatic cell nuclei in early embryonic development and persist in their activity for roughly half the time required to complete embryonic development.
Conclusion
Small RNAs occur in a variety of forms and play a central role in gene and genome regulation. Three specific classes of small RNAs—siRNAs, miRNAs, and piRNAs—are of particular interest in studying heritable adaptive mechanisms. These small RNAs are not only expressed and utilized by organisms to control their adaptation and healthy biological stasis, but they can also be transmitted in the sperm and egg cells to give the developing embryo a jump-start on adaptation.
These amazing little molecules are just one more component of the irreducibly complex systems in the cell that not only make life possible but enable creatures to thrive and fill the earth according to the grand design of the Creator who engineered them, the Lord Jesus Christ.
References
- Tomkins, J. P. 2023. Epigenetic Mechanisms: Adaptive Master Regulators of the Genome. Acts & Facts. 52 (4): 14–17.
- Cecere, G. 2021. Small RNAs in epigenetic inheritance: from mechanisms to trait transmission. FEBS Letters. 595 (24): 2953–2977.
- Mattick, J. and P. Amaral. 2023. Chapter 12: Small RNAs With Mighty Functions. In RNA, the Epicenter of Genetic Information. Boca Raton, FL: CRC Press.
- Tomkins, J. P. 2023. Transposable Elements: Genomic Parasites or Engineered Design? Acts & Facts. 52 (5): 14–17.
- O’Brien, J. et al. 2018. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Frontiers in Endocrinology. 9: 402.
- Ponsuksili, S. et al. 2021. Genetic regulation and heritability of miRNA and mRNA expression link to phosphorus utilization and gut microbiome. Open Biology. 11 (2): 200182.
- Wang, Y. et al. 2021. Sperm microRNAs confer depression susceptibility to offspring. Science Advances. 7 (7): eabd7605.
- Wu, X. et al. 2020. The Biogenesis and Functions of piRNAs in Human Diseases. Molecular Therapy: Nucleic Acids. 21: 108–120.
- Fabry, M. H. et al. 2021. Maternally inherited piRNAs direct transient heterochromatin formation at active transposons during early Drosophila embryogenesis. eLife. (10): e68573.
* Dr. Tomkins is Research Scientist at the Institute for Creation Research and earned his Ph.D. in genetics from Clemson University.