Evolutionists have long used the carbon-14, or radiocarbon, dating technique as a “hammer” to bludgeon Bible-believing Christians. A straightforward reading of the Bible describes a 6,000-year-old universe, and because some carbon-14 (14C) age estimates are multiple tens of thousands of years, many think that the radiocarbon method has soundly refuted the Bible’s historical accuracy.
However, these excessively long ages are easily explained within the biblical worldview, and 14C actually presents a serious problem for believers in an old earth. 14C has been detected in organic specimens (coal, wood, seashells, etc., containing carbon from formerly living organisms) that are supposedly hundreds of millions of years old—but no detectable 14C should be present in specimens that are even a little more than 100,000 years old! Nearly anyone can verify this for themselves using basic multiplication and division.
Radiocarbon Basics
Carbon comes in three “varieties” or isotopes: 12C, 13C, and 14C. Any carbon atom has six protons within its nucleus, but the different isotopes have different numbers of neutrons. In today’s world, only about one in a trillion carbon atoms is a 14C atom.
Cosmic rays (mainly high-energy protons) trigger a process in the atmosphere that changes atmospheric nitrogen into 14C. However, unlike the other two carbon isotopes, 14C is unstable and eventually decays back into nitrogen. The decay rate can be measured for a large number of these 14C atoms. Since this decay process slows as the number of 14C atoms decreases, it may be expressed best in terms of a half-life, which is the amount of time for half of any given sample of 14C to decay back into nitrogen. Thus, after one half-life, 50 percent of the original 14C atoms will remain. After two half-lives, 25 percent of the original 14C will remain, and so on. Today’s measured half-life of 14C is 5,730 years.
Because carbon is expected to be thoroughly mixed throughout the biosphere, atmosphere, and oceans, living organisms (which continually “take in” carbon throughout their lifetimes) are expected to have the same 14C/C ratio as the environment, or about one 14C atom per trillion carbon atoms. Once they die, however, organisms no longer take in new carbon, and the amount of 14C in their bodies begins to decrease.
In principle, this decay rate may be used to “date” the time since an organism’s death. But the calculated dates will only be accurate if the assumptions behind the method are correct.
Smallest Detectable Amount of Radiocarbon
Sensitive instruments called acceleration mass spectrometers (AMS) are used to count the 14C atoms within a sample of material. However, even the most sensitive AMS machines cannot detect fewer than one 14C atom per 100,000 trillion carbon atoms.1 Since the amount of 14C in a sample decreases with time, no radiocarbon at all should be detectable if the sample is sufficiently old.
The concentration of 14C (the number of 14C atoms per total number of carbon atoms) within a sample is indicated using a “percent of the 14C/C ratio in modern carbon,” or pMC notation. If a sample has one 14C atom per trillion carbon atoms, we would say that its concentration of 14C is 100 pMC, since this is 100 percent of the modern 14C/C ratio (one 14C atom per trillion carbon atoms). Likewise, one 14C atom per two trillion carbon atoms would be equivalent to 50 pMC.
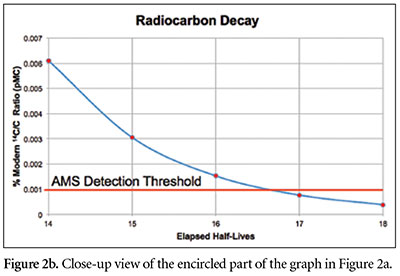
Since one 14C atom per trillion carbon atoms is equivalent to 100 pMC, then one 14C atom per 100,000 trillion carbon atoms is equivalent to 100 pMC/100,000 = 0.001 pMC. No instrument on earth can detect 14C in a sample whose 14C/C ratio is less than 0.001 pMC.2
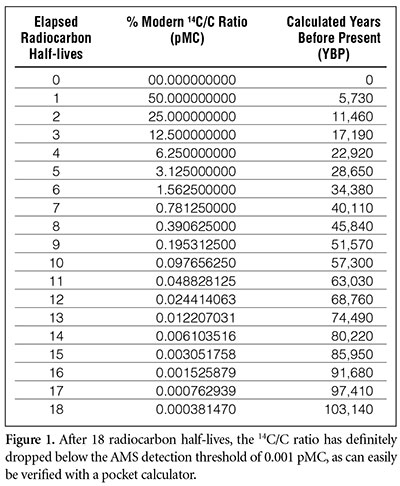
Assuming the initial value was 100 pMC, how much time will have transpired before the 14C/C ratio in a sample drops below 0.001 pMC?
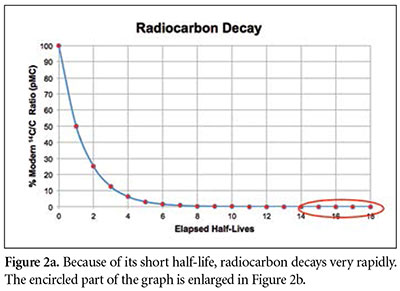
One can estimate this time by dividing 100 pMC by 2 repeatedly until the resulting number drops below 0.001 pMC. We find that about 18 such halvings are required for the pMC value to drop below 0.001 (Figures 1 and 2). (We could “round up” the value of 0.0007 pMC at 17 half-lives to 0.001 pMC, but the 0.00038 pMC at 18 half-lives is definitely below the detection threshold.) Since each half-life is 5,730 years, this means that no 14C at all would be detectable in a specimen that is older than about 18 × 5,730 years = 103,140 years.
 Dating Methods in Conflict
Dating Methods in Conflict
But researchers consistently detect 14C in samples thought to be tens of millions of years old. 14C has even been detected in diamonds, which some scientists claim are billions of years old! Radioisotope dating methods involving the heavier, longer-lived isotopes (methods such as uranium-lead, potassium-argon, etc.) are one of the main justifications that evolutionists use to argue for such vast ages. Because these radioisotope methods yield age estimates of many millions of years for igneous rocks, it is thought that sedimentary rocks are also millions of years old, as well as the organic remains found within them. Yet this assumption leads to a contradiction: If these organic samples really are many millions of years old, then they should be radiocarbon “dead.” But they aren’t!
Contamination?
 Evolutionists have attempted to blame these surprising results on a number of mechanisms.3 They often invoke “contamination” that occurred either in situ (on site in the earth) or during the radiocarbon testing process itself. However, the consistency with which 14C is found in these samples makes it difficult to argue that such results are all the result of in situ contamination. Moreover, diamond is extremely resistant to “natural” contamination by external 14C atoms.
Evolutionists have attempted to blame these surprising results on a number of mechanisms.3 They often invoke “contamination” that occurred either in situ (on site in the earth) or during the radiocarbon testing process itself. However, the consistency with which 14C is found in these samples makes it difficult to argue that such results are all the result of in situ contamination. Moreover, diamond is extremely resistant to “natural” contamination by external 14C atoms.
Furthermore, laboratories take great pains to keep contamination to a minimum, and researchers have found that, provided a sufficiently large testing sample is used (in the ballpark of 100 milligrams or so), the amount of such possible lab contamination is negligible compared to the 14C already present within the specimen.
 Finally, although contamination can sometimes occur, it should not be assumed in a particular instance unless there are good reasons to believe that it has. And a radiocarbon result that contradicts old-earth dogma is not a good enough reason by itself to invoke contamination!
Finally, although contamination can sometimes occur, it should not be assumed in a particular instance unless there are good reasons to believe that it has. And a radiocarbon result that contradicts old-earth dogma is not a good enough reason by itself to invoke contamination!
Assumptions…Assumptions
Instead of arbitrarily blaming these anomalous results on contamination, a far better (and more scientific) approach would be to question the correctness of the assumptions behind radioisotope dating methods.
One of these assumptions is that nuclear decay rates have always been constant. Although 14C decays fairly quickly, heavier isotopes (such as uranium-238) decay much more slowly. Because the present decay rates of these heavier isotopes are so small, the assumption that these rates have always been constant naturally leads to age estimates of millions and even billions of years.
Interestingly, however, some radioisotope methods tend to consistently yield younger age estimates than others, even when the techniques are used on the same rock units.4 Could this be a clue that radioisotope “clocks” might have “ticked” at different rates in the past, and that this variation in “ticking” is different for different radioisotopes?
If so, this would explain the discrepancy between the radiocarbon method and other radioisotope techniques. When today’s rates are used to calculate ages from certain radioisotope ratios, the results indicate that billions of years’ worth of nuclear decay of the heavier radioisotopes has occurred. But there is evidence that this decay occurred in accelerated “spurts,”5 which means the assumption that decay rates were always constant leads to age estimates that are much too high. This is the reason that 14C is still detectable in these “ancient” organic specimens—the specimens simply aren’t millions of years old! Furthermore, because the past variations in “ticking” were different for different radioisotopes, 14C did not experience as much accelerated decay as did the heavier radioisotopes. This is why the past episodes of accelerated decay did not completely eliminate the world’s 14C that existed before these episodes occurred.
Thus, although this is still an ongoing area of research,6 the presence of 14C within supposedly extremely “old” specimens is just one of several indicators of past accelerated nuclear decay.7
Why the High Radiocarbon Age Estimates?
Virtually all fossils found within sedimentary rocks are the remains of creatures that perished during the Genesis Flood about 4,500 years ago. Yet a skeptic might point out that the amounts of 14C found in these organic samples are smaller than what one might expect if they are only about 4,500 years old. And 4,500 years is less than one radiocarbon half-life, so from Figure 2 we might expect 4,500-year-old samples to have 14C/C concentrations greater than 50 pMC. Yet the 14C found within organic samples thought to date from the time of the Flood is generally only about 0.1 to 0.5 pMC. From Figure 1, a value of 0.098 ≈ 0.1 pMC corresponds to 10 half-lives, or about 57,000 years. Are these high radiocarbon “ages” a problem for the biblical worldview?
No. First, remember that no detectable 14C at all should be present within these samples if they really are millions of years old. Despite this apparent difficulty for the recent-creation view, this is, in fact, a much more serious problem for the old-earth view!
Second, such large calculated ages are based on the assumption that the 14C/C ratio has remained unchanged for tens of thousands of years.
A global flood like the one described in the Bible would invalidate this assumption. Creation scientists have estimated (based upon the amounts of organic matter thought to be contained within the sedimentary layers) that the carbon in the pre-Flood biosphere may have been 300 to 700 times greater than what is present in today’s world.8 Thus, the 14C/C ratio in the pre-Flood biosphere was hundreds of times smaller than today’s value.
A simple “thought experiment” illustrates why assuming a constant 14C/C ratio yields inflated radiocarbon ages. Suppose a time-traveling scientist journeys to the day before the Flood started (don’t worry; he’ll return before the Flood begins!) and radiocarbon-tests the remains of an animal that has just died. If the pre-Flood 14C/C ratio was 500 times smaller than today’s value, this would be equivalent to 100 pMC/500 = 0.2 pMC. This value of 0.2 pMC is very close to the value of 0.195 pMC found within Figure 1. About nine half-lives would have to elapse for a starting value of 100 pMC to decrease to 0.2 pMC. If the scientist did not realize that the pre-Flood 14C/C ratio was hundreds of times smaller than today’s value, he would calculate the animal’s age to be approximately 9 × 5,730 years = 51,570 years old—even though it had just died! Of course, he would realize that this age was nonsense, because he saw the fresh carcass. But if a scientist in the present did not have this firsthand knowledge and attempted to date the fossil remains of this very same animal (assuming it was fossilized during the Flood), he would conclude that the animal was 52,000—not 4,500—years old.
Thus, these “inflated” ages are not a problem for the biblical creationist, but the presence of detectable 14C in supposedly ancient organic specimens is a substantial problem for those who believe in an old earth.
References
- In scientific notation, 100,000 trillion is 1017.
- For technical details of the information in this article, see Baumgardner, J. 2005. Carbon-14 Evidence for a Recent Global Flood and a Young Earth. In Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative. Vardiman, L., A. A. Snelling, and E. F. Chaffin, eds. San Diego, CA: Institute for Creation Research and Chino Valley, AZ: Creation Research Society, 587-630.
- For example, evolutionists have attempted to explain that the 14C present in diamonds was caused by thermal neutrons within the earth’s interior. However, calculations show that this explanation doesn’t work (Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative, 614-616).
- Snelling, A. A. 2010. Radiometric Dating: Making Sense of the Patterns. Answers. 5 (1): 72-75.
- See chapters 2, 3, 4, and 7 in Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative.
- One apparent problem with episodes of accelerated nuclear decay is the enormous amounts of heat that would be generated—heat that would seemingly be fatal to life on earth. Since an alteration of nuclear decay rates may have been a miracle, God could have supernaturally dissipated this excess heat, and one respected creation physicist has proposed a mechanism for this. See Russell Humphreys’ discussion in Vardiman, L., A. A. Snelling, and E. F. Chaffin, eds. 2000. Radioisotopes and the Age of the Earth: A Young-Earth Creationist Research Initiative. San Diego, CA: Institute for Creation Research and Chino Valley, AZ: Creation Research Society, 369-373.
- Creationists believe that this accelerated nuclear decay likely occurred early in the creation week and during the Flood. See Radioisotopes and the Age of the Earth: A Young-Earth Creationist Research Initiative, and Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative.
- Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative, 617-620.
* Dr. Hebert is Research Associate at the Institute for Creation Research and received his Ph.D. in Physics from the University of Texas at Dallas.
Cite this article: Hebert, J. 2013. Rethinking Carbon-14 Dating: What Does It Really Tell Us about the Age of the Earth? Acts & Facts. 42 (4): 12-14.