The March 1998 Impact article"Cloning - What is It and Where is It Taking Us?" discussed the procedure of cloning by somatic cell transfer. In that procedure, the nucleus from a cell derived from an embryo, a fetus, or tissue of an adult is inserted into an egg from which the nucleus has been removed. After the egg develops to the appropriate stage, the embryo is inserted into the uterus of a properly prepared female and allowed to develop to term. This produces an offspring essentially genetically identical to the animal that provided the nucleus that was inserted into the egg. This article will discuss the transfer of genes from one species to another in order to endow the recipient species with beneficial properties, or to enable the recipient to produce human proteins for injection into human patients who lack a vitally important protein.
Production of Therapeutic Proteins by Gene Transfer
There are many proteins essential to good health that some people cannot produce because of genetic defects. These proteins include various blood-clotting factors causing hemophilia, insulin (resulting in diabetes), growth hormone (resulting in lack of proper growth), and other proteins, the administration of which corrects pathological conditions or results in other therapeutic benefits. The early work in this field employed bacteria. Some bacteria in a bacterial culture may contain small circular DNA molecules called plasmids. These plasmids are not part of the chromosomal DNA that is possessed by all the bacteria of the culture. As these exceptional bacterial cells reproduce by binary fission or cell division, the plasmids are transmitted to the daughter cells. They can also be transmitted to other cells by conjugation. Scientists have learned how to utilize plasmids to transfer human genes to bacterial cells. If the gene inserted into the plasmid of bacteria is the human gene for insulin, for example, the bacteria into which this gene is inserted produces human insulin.
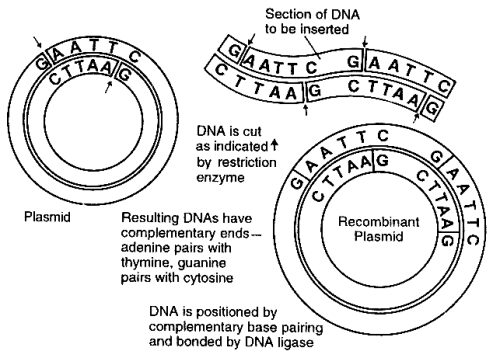 |
Scientists have identified and isolated enzymes (called restriction enzymes, or restriction endonucleases) each of which cuts genes in very specific places. More than 100 of these enzymes have been isolated. After the position of the human gene that codes for the desired therapeutic protein has been located on the chromosome, the gene is cut out using the appropriate restriction enzymes and the gene is isolated. The same restriction enzymes are used to cut out a piece of the circular DNA plasmid. Thus, the two ends of the human gene will be those that will link up with the open ends of the plasmid. An enzyme called DNA ligase is used to couple each end of the gene to the open ends of the plasmid, restoring a circular DNA molecule with the human gene replacing the piece cut out of the plasmid. These plasmids, now including the human gene, are reinserted into bacteria. These bacteria are cultured, producing large quantities of identical bacteria carrying the human gene that is reproduced along with the bacterial DNA. Furthermore, these bacteria produce the human protein coded for by the spliced human gene. The protein is isolated from the bacterial culture, purified, and injected into those patients suffering from pathological conditions because their bodies cannot produce sufficient quantities of the protein.
Before genetic engineering, these proteins had to be painstakingly isolated from tissues or blood, but since they are produced in such minute quantities, the isolation of significant quantities required the processing of large quantities of material. As a result, they were very expensive. Relatively much larger quantities were obtained from genetically altered bacterial cultures, but the cost, although less, was still high. The bioreactors (that is, the machinery required to culture large quantities of the bacteria containing the human gene) are enormously expensive and must be operated by several scientists and technical assistants. Furthermore, the proper operation of the bioreactor is sensitive to small changes in temperature and composition of the culture broth.
Fortunately, an alternative method has been developed which promises to be much less expensive and much more efficient. This method utilizes an animal, such as a pig, as the bioreactor. It required years for scientists to design, develop, and build the very expensive, difficult to operate bioreactor devised by man. God had already devised a much more efficient, much cheaper bioreactor. Scientists finally realized that it would be possible by genetic manipulation to induce a female pig, cow, or other animal to produce the desired human proteins in its milk.
 |
This procedure has now been successful in both pigs and cows. Among animals, the pig has a number of advantages. Its gestation period is only four months. At 12 months of age the pig is fertile and produces large litter sizes (usually 10 to 12 piglets). A lactating pig produces 300 liters (about 315 quarts) of milk in a year. The procedure is carried out as follows:
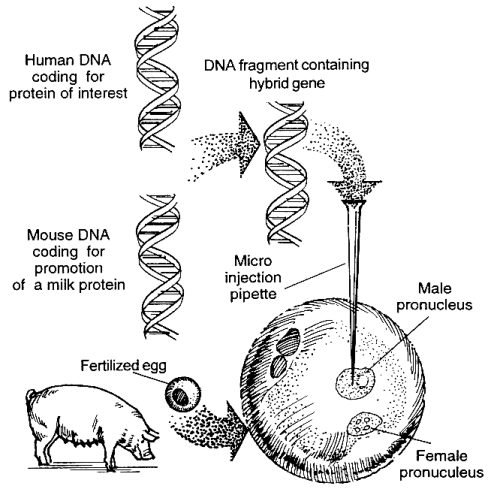
- The DNA fragment (gene) that codes for the needed human protein is isolated.
- The DNA fragment that promotes production of proteins in mammary glands is isolated and linked or combined to the human gene.
- Fertilized eggs from a donor pig are obtained.
- The human DNA is injected into an egg in the region of the male pronucleus (DNA from the sperm before it unites with DNA of the egg) using a very slim micro pipette. The human DNA is thus incorporated into the pig nuclear DNA.
- The egg is implanted in the uterus of another pig and develops into a newborn female pig.
- The desired protein is isolated from the milk of the female pig once grown.
This procedure was carried out successfully by American scientists and the results were published in 1994.1 The human gene they used codes for Protein C that acts to control blood clotting. They obtained one gram of Protein C from each liter of milk produced by the pig. This is 200 times the concentration found in human blood plasma. Only about one third of the Protein C obtained was biologically active. The reason for this is that many modifications of a protein must be performed in a cell after the protein chain is formed. For example, sections are cut out of the protein; complex sugar groups may be attached at certain places in the protein chain; and cell wall anchor groups may be added. The scientists discovered that a key processing enzyme, called furin, was present in insufficient quantities, so they added to their gene complex the gene that codes for furin. This increased the yield of active Protein C. The human proteins produced in this way must be tested for safety and effectiveness. At this writing, an anti-clotting protein called anti-thrombin is now being tested in clinical trials.
Comparing this procedure to the use of bioreactor machines illustrates the fact that a generation of biochemical engineers failed to match the abilities of a tool for making proteins (the pig) that God had prepared. The mammary gland is optimized to maintain a high density of cells to deliver to them an ample supply of nutrients and to channel proteins that are produced in a form that can easily be isolated and purified. This procedure has proven to be successful and promises to provide a method for producing valuable therapeutic proteins at much lower cost.
1 R.M. Akers, et al., in Recombinant DNA Technology, vol. 2: Special Issue of Annals of the New York Academy of Science, vol. 721, pp. 218-233, May 2, 1994. W.H. Velander, H. Lubon, and W.N. Drohan, Scientific American, January 1997, pp. 70-74.
* Dr. Gish is Vice President of ICR. He has a Ph.D. in biochemistry from the University of California, Berkeley, and worked for many years in research at the Upjohn Company.